Exploring Perovskites: A Conversation with Marina Leite
Though their optical and electrical properties are very promising, perovskites are a class of materials that are still understudied. Until researchers have a better understanding of their overall properties, they can’t learn how to control them to create ubiquitous devices like solar cells, LEDs and photodetectors.
Materials science and engineering (MSE) professor Marina Leite aims to help change this. Her group looks at new ways to discover the properties of perovskites, including atomic force microscopy (AFM) and machine learning. Two of their recent publications, which survey the latest research on using AFM to probe halide perovskites, made journal covers in Advanced Energy Materials and Advanced Materials Interfaces in July and August 2020, respectively.
The MSE department asked Leite a few questions to learn more:
MSE: What are halide perovskites and why are they such a popular topic in materials science today?
Marina Leite: "Halide perovskites are a burgeoning class of material for optoelectronic devices. Specifically, they can be used to make solar cells that are high-efficiency and low-cost. In the last decade, they have attracted enormous attention from the materials science, chemistry and physics communities because they have promising optical and electrical properties, are inexpensive to fabricate and easy to scale up.
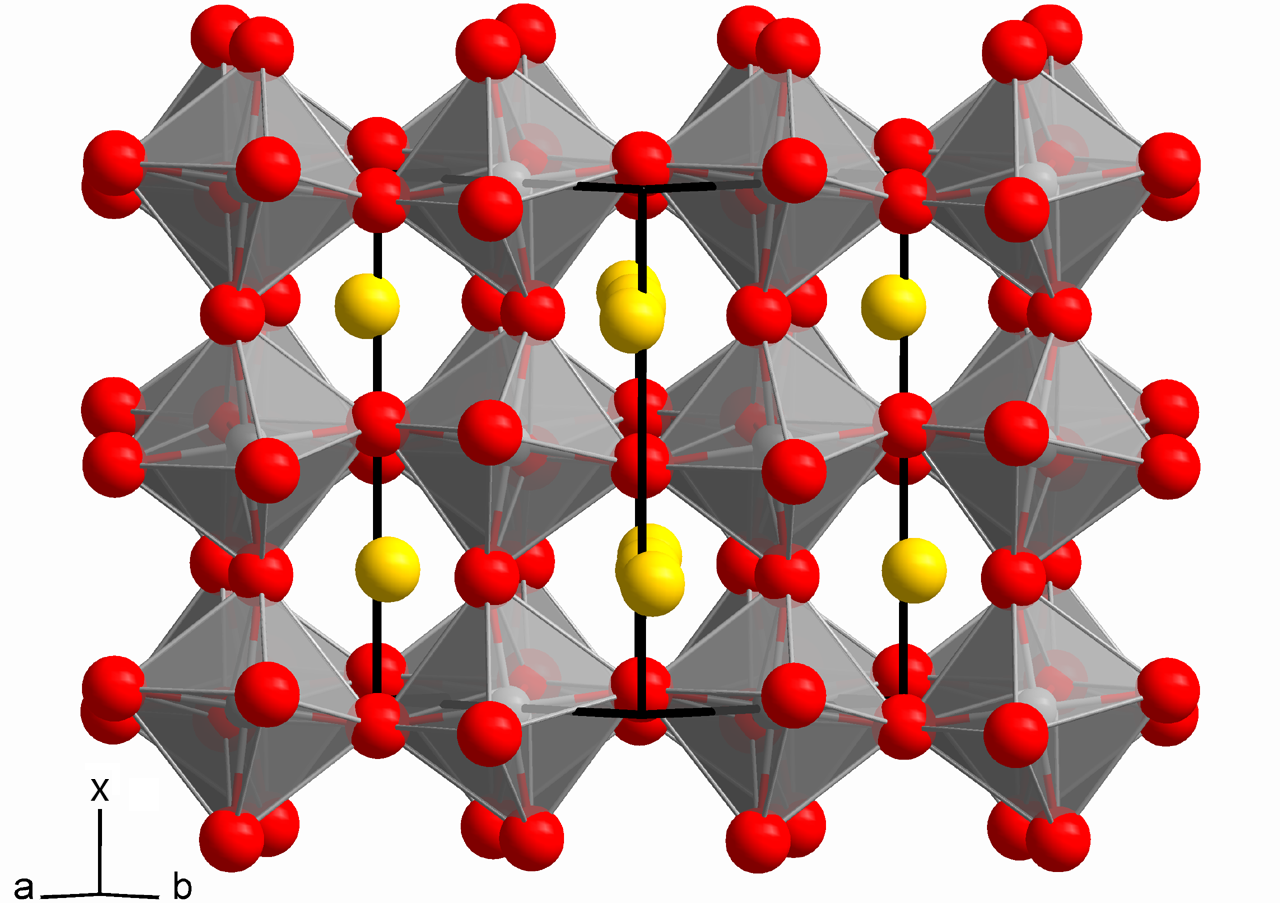
"Their ABX₃ crystal structure is composed of organic and inorganic anions and cations, and layers of these materials are composed of nano- or micro-scale crystals. This combination of ions/molecules is key for efficient charge carrier (electrons and holes) generation, recombination and transfer. Metal halide perovskites are especially unique because in their ABX₃ structure, A and B are cations and X is a halide anion, usually iodine, bromine or chlorine. This makes them inexpensive to fabricate while presenting excellent electrical properties."
What is AFM and why is it such a powerful tool to study perovskites?
"When we gently glide along the surface of an object with the tip of our index finger, we feel its texture, shape, temperature, hardness, etc. AFM is very similar, but instead of using our fingertip, we use a nanoscale probe. It provides nanoscale spatial resolution in three dimensions, which gives us a full picture of the material properties and enables us to quantify the differences between the distinct grains that make up the perovskite layer.
"AFM is widely used to explore the structure of nanoscale materials, but it has been under-used to investigate the dynamic behavior of halide perovskites. Its ability to measure the morphological, electrical, magnetic, mechanical, chemical, and optical properties of materials is crucial for understanding and controlling the physical and chemical phenomena that governs them, including light absorption, charge carrier transport, and ion motion."
Your group recently published a review paper in Advanced Energy Materials on this topic. How do you hope it will impact the field?
"Our review paper surveys a series of recent scientific findings related to metal halide perovskites physical and chemical properties, and how AFM has enabled the discovery of new phenomena in this material. Though we focused on metal halide perovskites, the techniques we discuss can and should be expanded to other types of materials currently being developed for renewable energy systems."
What's the next step for you and your group in this area?
"There are several open questions related to this incredibly rich system that might be answered by visualizing halide perovskites’ dynamic processes at the nanoscale. For example, how do cations such as caesium and rubidium affect the electrical transport properties of the grains and its boundaries? Or, can light-induced treatments suppress ion motion within the perovskite layer, and can this be used to improve the photovoltage of perovskite solar cells? I am excited to see our group leverage our expertise in AFM to advance this knowledge!"
Read the Advanced Energy Materials publication to learn more.
